Revolutionizing Endoscopic Treatment
Empowering endoscopists to transform lives through innovative, minimally invasive solutions for GERD, Obesity, and Diabetes
From Fritz Haller, CEO, Endolastic
Endolastic Q2 2025 Investor Update
Credibility Proven. Scalability Unlocked
Investment Snapshot
Endolastic is raising a $4M physician-led seed round:
- $515,000 already committed
- SAFE at $20M post-money valuation
- Minimum investment: $25,000
Funds support commercial launch, regulatory filings, and expansion into diabetes. We now have clinical data, KOL backing, and a differentiated platform built for real-world impact.
New Advisory Board Addition: Dr. Todd H. Baron
We’re honored to welcome Dr. Todd Baron, Director of Advanced Therapeutic Endoscopy at UNC Chapel Hill, to our Scientific Advisory Board. Dr. Baron is one of the world’s most accomplished interventional endoscopists with over 600 peer-reviewed publications, lead editor of the definitive ERCP textbook, and recipient of the 2023 Rudolf V. Schindler Award—the highest honor in GI endoscopy. His clinical focus spans the entire GI tract, making him an ideal partner to help guide Endolastic across GERD, obesity, and diabetes.
Clinical Progress
- 50+ successful human cases completed with Gen 1 EndoBand™
- 85%+ GERD
symptom resolution via Anti-Reflux Band Mucosectomy (ARBM)
By comparison, the leading alternative—TIF (Transoral Incisionless Fundoplication)—shows GERD symptom resolution in the 68–75% range across most published series.1 - 20%+ weight loss in
Endoscopic Band Gastroplasty (EBG) cases
For reference, Boston Scientific’s OverStitch-based ESG (Endoscopic Sleeve Gastroplasty) reports weight loss in the 15–18% range at 12 months.² - Zero serious adverse
events (SAEs) reported
By contrast, TIF for GERD and ESG for obesity—two leading endoscopic procedures—report SAE rates of 2.3–2.4%.³⁻⁴
EndoBand™ has demonstrated zero SAEs across 50+ human cases, underscoring both safety and simplicity.
New Data Highlight:
Dr. Mohamed Abeid’s latest peer-reviewed study (Obesity Surgery, May 2025) explores antral application of Gen 1 EndoBand™. Among 12 women treated, the antrum-targeted group lost an average of 16.2% total body weight in just 3 months, outperforming the gastric body group in efficacy, tolerability, and procedural simplicity.
Clear Difference: Why Gen 2 EndoBand™ Matters
Gen 1 proved the concept—Gen 2 powers the scale:
- Wider & deeper tissue capture enables engagement of the muscularis propria, promoting more durable anatomical reshaping
- Calibrated pressure control ensures safe, consistent ischemia and necrosis
- Engineered elastomeric bands are timed to hold tissue long enough for regeneration—without excessive pressure that could risk transection or perforation
With these enhancements, Gen 2 EndoBand™ transitions from KOL use to everyday endoscopy—safe, simple, and repeatable in ASC and outpatient settings worldwide.
Next Milestone: Type 2 Diabetes Clinical Trial
Launching this quarter in Santiago, Chile, our first band-assisted DMR (Duodenal Mucosal Resurfacing) trial targets glycemic control through mucosal regeneration. This 20-minute outpatient procedure requires no general anesthesia, offering an anatomical reset for diabetes—without drug dependence or high-risk surgery.
Why Now: GLP-1 vs Bariatric Surgery vs EndoBand
A 2025 NYU Langone study (ASMBS, June 2025) compared GLP-1 drugs to bariatric surgery across over 14,000 patients:
- Bariatric surgery: ~25.7% weight loss, -0.5 HbA1c
- GLP-1 drugs: ~5.3–7.6% weight loss, -0.2 HbA1c
EndoBand™ sits between them: an anatomical solution with surgery-like durability—but with outpatient simplicity and fractional cost. We're not burning, stapling, or medicating—we’re restoring.
Let’s Reshape What’s Possible—Together.
Best regards,
Fritz Haller
CEO | Endolastic, Inc
Endolastic.com
References
- Trad et al., 2020; Hakansson et al., 2021
- Jirapinyo et al., 2022; Sharaiha et al., 2021
- Efficacy and Safety of TIF: PMC8162865
- ESG Meta-Analysis: PubMed 30388381; GIE Journal, 2021
1 Platform. 3 Metabolic Conditions.

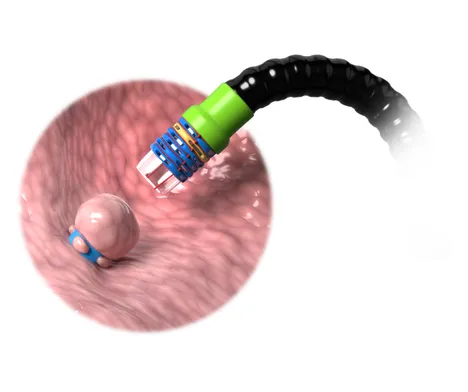
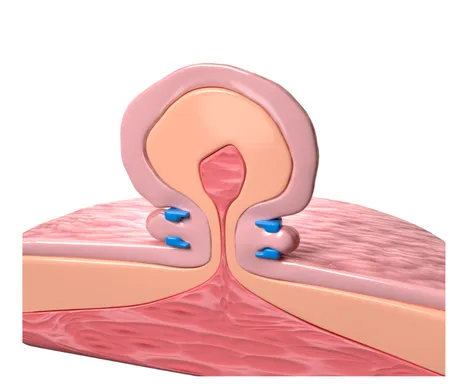
Ligation Based Platform
EndoBand utilizes Endolastic™ technology to deliver versatile and precise support for tissue management, empowering professionals with a minimally invasive, reliable solution.
GERD
Anti-Reflux Band Mucosectomy (ARBM)
Our Endolastic™ technology provides a minimally invasive solution for chronic acid reflux, offering long-term relief without the need for ongoing medication.
Obesity
Endoscopic Band Gastroplasty (EBG)
Endolastic's innovative approach helps patients achieve sustainable weight loss through a quick, outpatient procedure with minimal recovery time.
Type 2 Diabetes
Duodenal Mucosal Resurfacing (DMR)
By addressing obesity, our technology aids in managing type 2 diabetes, potentially reducing medication dependence and improving overall health outcomes.
The Endolastic™ Difference
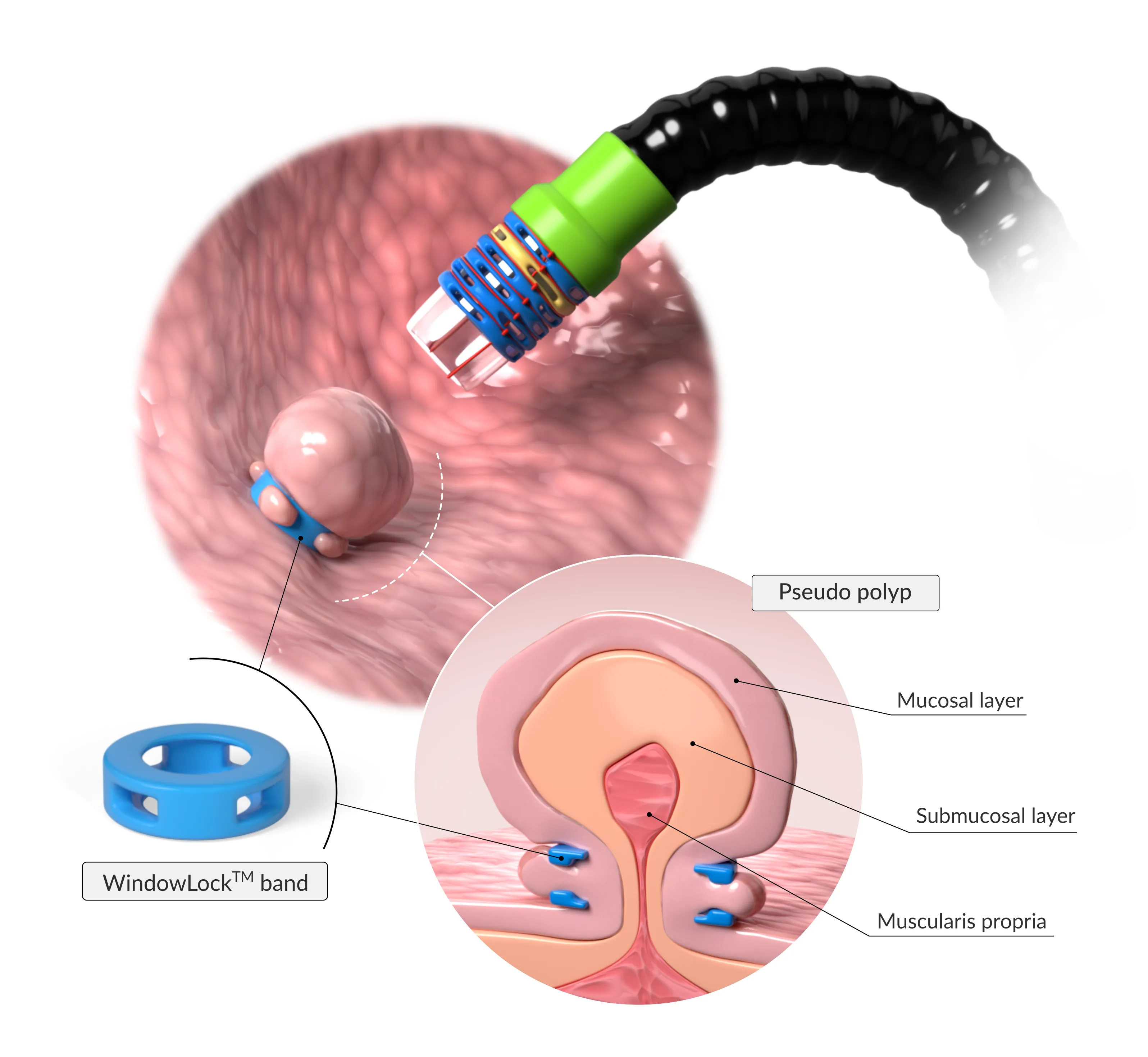
- Minimally invasive ligation based procedure
- MAC Sedation enables ASC patient access
- Treats the source, not the symptom
- Targets dysfunctional GI tissue
- Quick recovery time
- Customizable for various applications
- Natural tissue regeneration
- Repeatable and adjustable
How Endolastic Platform Works
Minimally Invasive
Endolastic's WindowLock™ bands are applied endoscopically, meaning no incisions are needed.
- Low Risk of Complications: Reduced risk of infection and complications, with no incisions required.
- Outpatient Procedure: Can be performed in an outpatient setting.
Quick Procedure
Endolastic procedures typically takes less than 30 minutes, with minimal recovery time.
- Quick Recovery: Patients experience faster recovery times compared to surgical procedures.
- Patient Comfort: Conscious sedation reduces the need for general anesthesia.
Effective Results
Patients experience significant improvement in symptoms, often within days of the procedure.
- Natural Tissue Regeneration: The innovative ligation based pressure zones stimulate the body's natural healing processes.
- Repeatable: The procedure can be safely repeated if necessary.
Contact Us
For Distributors
Join Our Network
We are actively assessing strategic partnership opportunities with a select group of distributors, as part of our comprehensive global go-to-market strategy, encompassing both direct and distribution-led channels.
- High-demand, innovative products
- Significant market potential in GERD, obesity, and diabetes
- Cost-effective solutions with high margins
- Easy to demonstrate and train physicians
- Complements existing endoscopic product lines
- Potential for recurring revenue through disposable components